Systems
Complex biomolecular systems, including intrinsically disordered proteins. Bioactive and drug-like compounds targeting selected functional macromolecules.
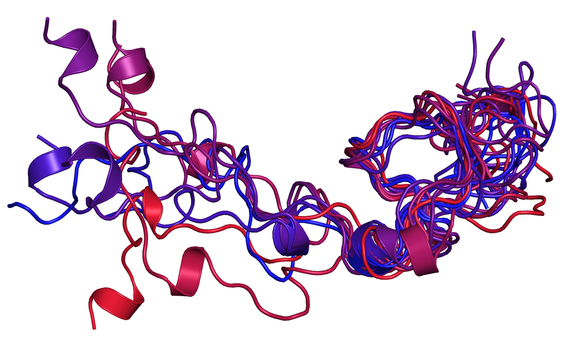
Intrinsically disordered proteins
Intrinsically disordered proteins (IDPs) and regions (IDRs) have a key role in regulation and signaling mechanisms.
• NUPR1 is an oncogenic protein with a totally disordered structure. Its function can be inhibited by using a variety of molecular species that include small drug-like compounds, peptides, and dendrimers. However, NUPR1 remains unfolded even in interaction with these molecular species, as well as under crowding conditions and in partnership with other proteins.
• NUPR1L is a paralogue of NUPR1 also involved in DNA repair. However, after emerging from the duplication of the same ancestral coding gene, the two proteins diverged to perform some different functions. Compared to NUPR1, it has a longer sequence with more hydrophobic residues, possesses some flickering structure, and is able to self-associate into oligomers.
• Nuclear localization signals/sequences (NLSs) are short protein regions acting as a recognition signal within cargo proteins that need to be transported from the cytoplasm into the nucleus. The conformational propensities and binding features of peptides corresponding to NLSs reveal information on molecular recognition processes that are vital for cells.
Enzymes
Enzymes are proteins performing catalytic reactions, and control chemical processes essential for any living species.
• PADI4 belongs to a family of five enzymes catalyzing the post-translational modification of selected arginine residues to citrulline. This protein is the sole member that has been detected both in the cytoplasm and the nucleus under ordinary conditions, and it is able to interact with a variety of other proteins.
• The main protease 3CLpro/Mpro of SARS-CoV-2 is an enzyme that is crucial for the replication of the coronavirus. It oligomerizes in increasingly complex assemblies (i.e., starting from the monomeric species it can form dimers, tetramers, octamers, etc.), and its function can be inhibited by targeting it with small drug-like compounds.
Transport proteins
Transport proteins are in charge of shuttling selected molecular species within living organisms.
• Karyopherins are proteins devoted to the transport of cargo macromolecules between the cytoplasm and the nucleus in eukaryotic cells. In particular, importin α3 is a shuttle protein that has a larger flexibility compared to other members of the same protein family, and it is highly conserved across different species.
• Albumin is one of the most studied macromolecules, because it is the most abundant protein in human blood. It binds and provides the transport of fatty acids and other endogenous substances, as well as of exogenous substances that include a large variety of administered drugs and of nutraceutical substances.
• The model transport protein β-lactoglobulin is well studied especially in food chemistry, since it is the most abundand protein in the milk of cows and many other mammals, with the notable exception of humans. This protein is a carrier of fatty acids, and of other compounds of interest in nutraceutics.
Estrogen receptors
Estrogen receptors are proteins activated by the hormone estrogen, and also targeted by some other (agonist/antagonist, or mixed type) compounds.
• GPER is an estrogen-sensitive protein belonging to the G protein-coupled receptors (GPCRs), which form a large family of membrane surface sensors that detect molecules and activate cellular responses as a consequence. This protein can interact with several ligands, including small compounds and larger peptides.
• The classical estrogen receptors ERα and ERβ are nuclear transcription factors involved in the regulation of many complex physiological processes that are fundamental for human health. They may interact not only with physiological estrogens, but also with other estrogen-mimicking small molecules, including some of natural origin.
Blue copper proteins
Blue copper proteins (or cupredoxins) owe their name to their intense color due to a copper ion in the active site, and are electron transfer agents in redox reactions.
• Azurin is a small periplasmic blue copper protein of bacterial origin, possessing some remarkable electron transfer features. In addition to its redox properties, azurin has also been found to have anticancer properties through its interaction with the tumor-suppressor protein p53, known as the "guardian of the genome".
• Amicyanin is a cupredoxin found in some bacteria, which differs from its closest related proteins of the same family for having an additional region at its N-terminal end. It acts as an electron-accepting intermediate in a three-member protein redox complex. Therefore, it is used as a model for the study of interprotein electron transfer reactions.