Research
Structural, dynamical, and functional features of biomolecules. Protein folding/unfolding/misfolding, and 'non-folding'. Binding properties in host-guest molecular complexes.
Protein folding/unfolding/misfolding, and 'non-folding'
Protein folding is the complex process that most polypeptides undergo after their synthesis, leading to a distinct functional conformation. Such reaction can be reversed (i.e., unfolding), or get stuck in inactive intermediate species (i.e., misfolding). Some proteins do not require an ordered structure to function, and remain in a disordered state (i.e., 'non-folding').
These processes depend not only on the features of the specific protein sequence, but also on the environment, including the solvent and the other molecular species in solution: cosolvents, osmolytes, cofactors, active binding partners, or inert crowding agents. Denaturing effects can also be induced by temperature, pressure, and external mechanical forces.
Understanding protein folding, or the other related processes described above, is one of the biggest challenges in molecular biophysics. It requires a combined effort of experimental and computational methods to overcome to the difficulties of observing such delicate phenomena, and the help of theoretical models to summarize the results into a single unified picture.
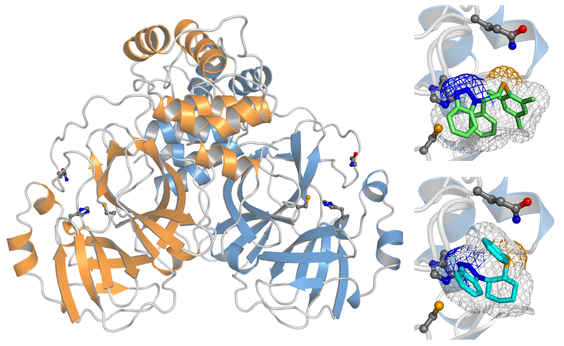
Protein-protein interactions
Protein-protein interactions (PPIs) play a primary role in biology, and dictate the function and specificity of the polypeptides involved, as well as their structural evolution in the long run. The large diversity of PPIs found in living organisms requires a concerted approach of experimental and theoretical technique to investigate them.
Molecular complexes may be homo-oligomeric or hetero-oligomeric. Furthermore, the lifetime of the complex is also controlled by PPIs, therefore both transient and permanent complexes can form, sometimes depending on subtle changes in the macromolecules involved, or determined by specific molecular triggers that shift the oligomeric equilibrium.
Computer simulations can help to clarify these aspect, by predicting or modeling the supramolecular assembly of a protein-protein complex, and providing an estimate of the binding free energy that ultimately determines the formation of such a complex. Due to the large size of the protein chains involved in the association, these simulations are particularly intriguing.
Binding properties in host-guest molecular complexes
Interactions between macromolecules and ligands are responsible of recognition, association, transport, and release of molecular species that are crucial from a functional point of view. These mechanisms are involved in many biological processes that are vital for every living organism, and can be exploited in several pharmaceutical and nanotech applications.
Molecular simulations can be used to determine a model of interaction between a macromolecule and a variety of ligands. Structural and dynamical properties driving the function of a molecular complex can be predicted; for instance, those involving small inorganic compounds that may include molecules of physiological or pharmacological interest.
Expertise on ligand binding processes is especially important in the field of drug discovery and design, which is a challenging and yet crucially important scientific and industrial area. In particular, rational drug design requires a multidisciplinary approach that combines different and sophisticated experimental and computational tools.